Cláudia Leite graduated in Biochemistry and holds a PhD in Molecular and Cell Biology from the University of Porto. During her PhD, she investigated how mitochondria adjusts their activity to regulate cell cycle progression in budding yeast. She has recently joined the Coffer lab as a postdoctoral researcher to investigate the role of nuclear acetyl-CoA-producing enzymes in regulating transcriptional reprograming.
Lab day out: canoeing in Holland's beautiful 'green heart'
CLICK PHOTO TO VIEW FULL SIZE
Paper: Nuclear metabolism controls chromatin remodelling during T cell activation
Extremely happy to have our study exploring nuclear metabolism in the control of chromatin remodeling published (link below). This became a huge project driven by Enric Mocholi and wouldn't have been possible without generous collaborations from groups in the EU, USA and Canada (through lockdown). A BIG thank you to all involved.
In brief
After T cell activation, histone acetylation and transcriptional reprogramming require glycolysis and the pyruvate dehydrogenase (PDH)-dependent production of extramitochondrial acetyl-CoA. Here we show that PDH translocates to the nucleus close to chromatin-remodeling complexes, highlighting how metabolic and histone-modifying enzymes cooperate in regulating T cell activation.
Highlights
- PDH is required for histone acetylation and transcription after T cell activation
- MPC1 and ACLY are not required for T cell activation and transcriptional reprogramming
- T cell activation leads to PDH nuclear translocation close to chromatin-remodeling complexes
Lab Jeu de Boules night out
The Winners!
Congratulations Dr. Anita Govers!
Many congratulations to Dr. Anita Govers who successfully defended her thesis "Epigenetic regulation of normal and aberrant myelopoiesis: a balancing act". It has been a long journey and super happy that we could celebrate this milestone together with co-promoter Dr. Marije Bartels. Many thanks to the thesis committee Prof. Marry van den Heuval-Eibrink, Prof. Femke van Wijk, Prof. Jurgen Kuball, Prof. Roland Kuiper, Prof. Joop Jansen en Dr. Marc Bierings.
In the last two decades, epigenetic changes have become more and more evident as a contributing factor in aberrant myelopoiesis, however the exact roles are yet poorly understood. Furthermore, knowledge about epigenetic regulation of normal hematopoiesis is far from complete. Nonetheless, the use of chromatin modifying drugs such as HDACi, has rapidly increased in the past years.
Work in this thesis is aimed to increase understanding the effects of epigenetic regulation and epigenetic modifiers on both normal and aberrant hematopoiesis.
Dutch Society for Immunology Meeting 2022
Great poster and oral presentations from Alessandro, Sonia and Enric at this years Dutch Society for Immunology meeting.
Colorectal Cancer-Infiltrating Regulatory T Cells
Here, we review our understanding of the role of T regulatory (Treg) cells in colorectal cancer (CRC), the possible mechanisms that support their homeostasis in the tumour microenvironment, and current approaches for manipulating Treg cells function in cancer.
Colorectal cancer (CRC) is a heterogeneous disease with one of the highest rates of incidence and mortality among cancers worldwide. Understanding the CRC tumor microenvironment (TME) is essential to improve diagnosis and treatment. Within the CRC TME, tumor-infiltrating lymphocytes (TILs) consist of a heterogeneous mixture of adaptive immune cells composed of mainly anti-tumor effector T cells (CD4+ and CD8+ subpopulations), and suppressive regulatory CD4+ T (Treg) cells. The balance between these two populations is critical in anti-tumor immunity. In general, while tumor antigen-specific T cell responses are observed, tumor clearance frequently does not occur. Treg cells are considered to play an important role in tumor immune escape by hampering effective anti-tumor immune responses. Therefore, CRC-tumors with increased numbers of Treg cells have been associated with promoting tumor development, immunotherapy failure, and a poorer prognosis. Enrichment of Treg cells in CRC can have multiple causes including their differentiation, recruitment, and preferential transcriptional and metabolic adaptation to the TME. Targeting tumor-associated Treg cell may be an effective addition to current immunotherapy approaches. Strategies for depleting Treg cells, such as low dose cyclophosphamide treatment, or targeting one or more checkpoint receptors such as CTLA-4 with PD-1 with monoclonal antibodies, have been explored. These have resulted in activation of anti-tumor immune responses in CRC-patients. Overall, it seems likely that CRC-associated Treg cells play an important role in determining the success of such therapeutic approaches.
Full access to review paper here
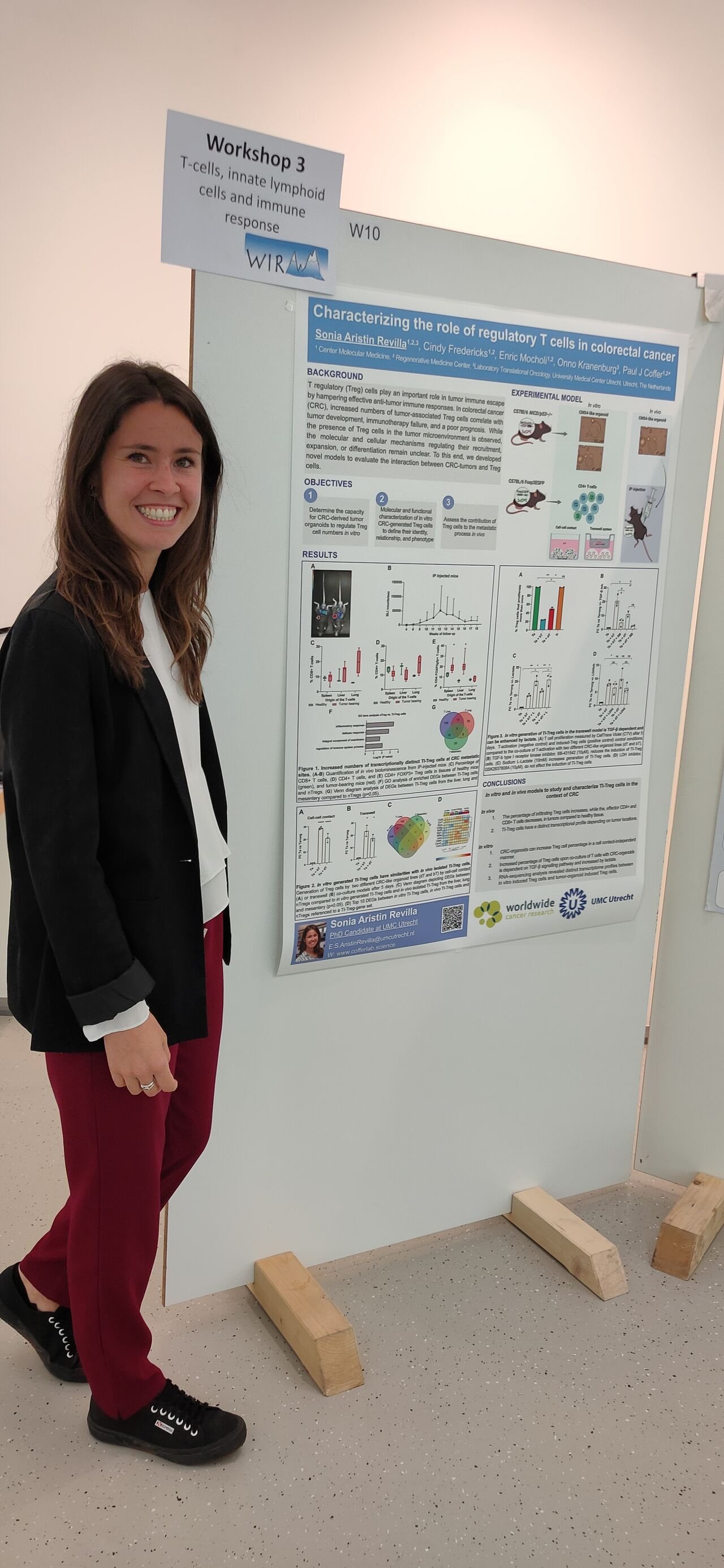
Immunology in the Swiss mountains: WIRM meeting
The World Immune Regulation Meeting (WIRM) takes place in Davos, Switzerland. Coffer Lab PhD students Sonia Aristin and Alessandro Cutilli were both selected to present their projects in oral presentations and poster sessions. Sonia discussed her work on characterising the role of regulatory T cells in colorectal cancer. Alessandro presented his data on the effects of chemotherapy-induced damage of the intestinal epithelium on T cell homeostasis. A great offline experience with more than 400 participants. Great job guys!!